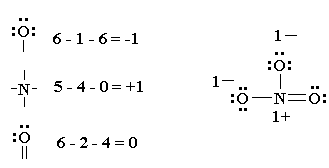Best Info About How To Draw A Lewis Structure
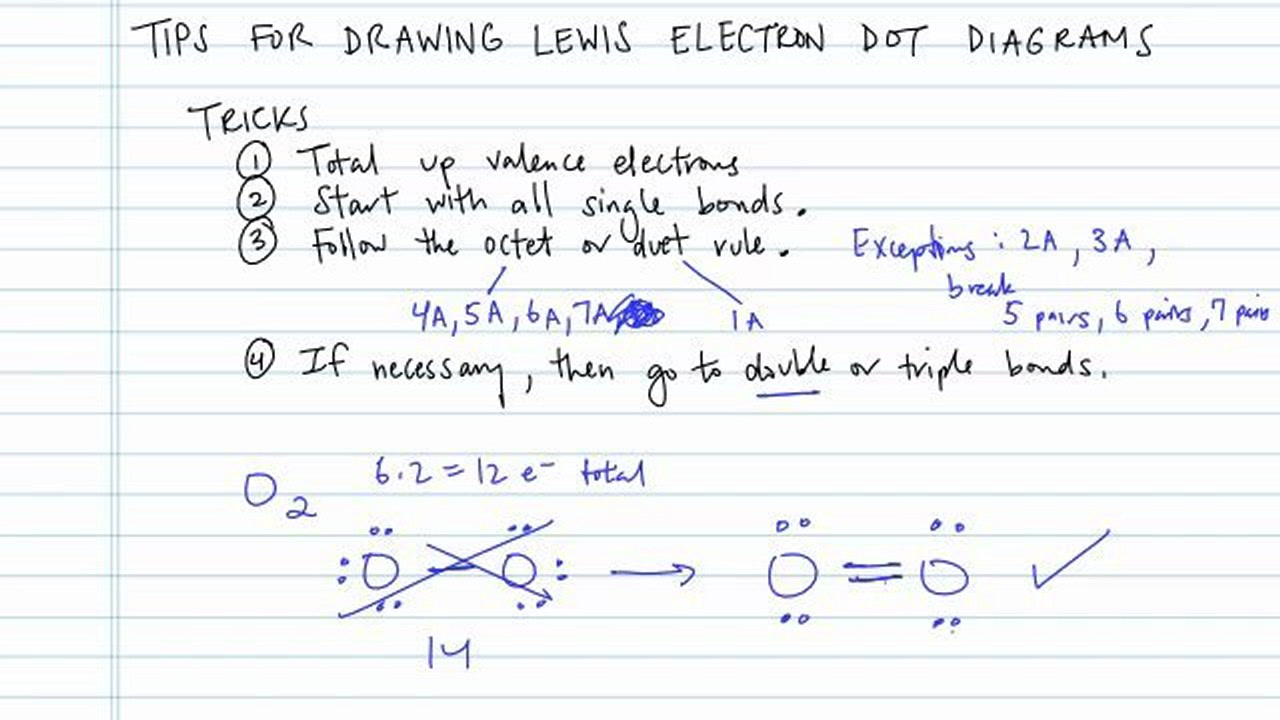
The lewis structure is drawn for individual atoms by putting a dot for each available valence electron around the atom.
How to draw a lewis structure. A lewis diagram shows how the valence electrons are distributed around the atoms in a molecule. Remember that lewis dot structures only give reasonable results for covalent compounds. Determine the total number of valence electrons in a molecule.
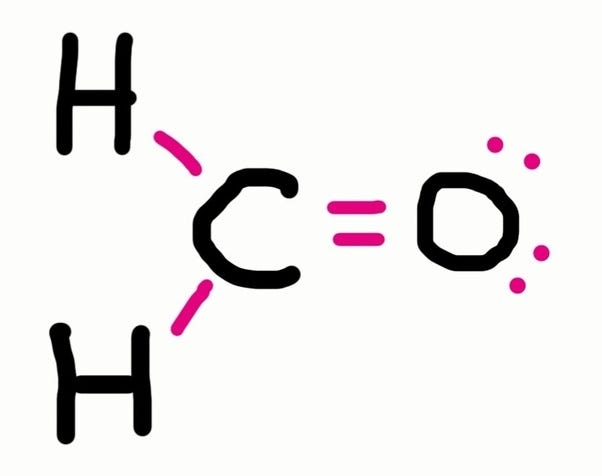
How to draw lewis structures 1. Lewis structure of oxygen \(\left( {{{\rm{o}}_{\rm{2}}}} \right)\). We will put c atoms in.
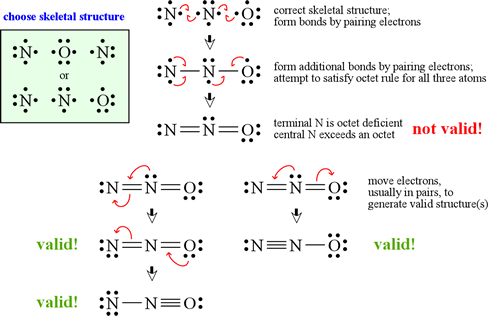
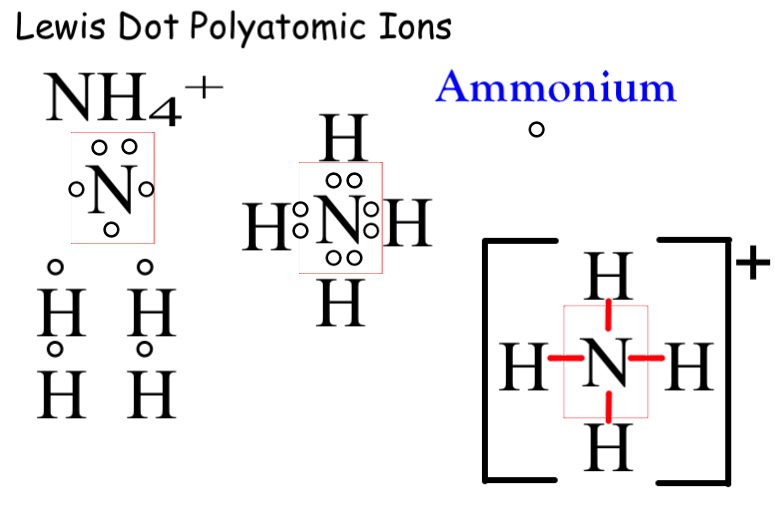
In order to draw a lewis structure for c 2 {_2} 2 h 4 {_4} 4 , first we will count the total number of valence electrons, which is 12 (8 from c atoms and 4 from h atoms). How to draw lewis structure for large compounds? Lewis structures for polyatomic ions.
Count the total number of. The following is a guide for drawing correct lewis structures of more complex molecules, with bonding pairs represented by short lines. Put the least electronegative atom in the center.
Five basic steps of drawing lewis structure in this section would follow this. Shared pairs of electrons are drawn as lines between atoms,. Drawing if lewis structure is as simple as lewis structure of other compounds.
The steps to draw the lewis structures of various types of compounds are given below: This chemistry video provides a basic introduction into how to draw lewis structures of common molecules such as cl2, o2, of2, ch4, nh3, h2o, c2h2, and n2h4. Count total valence electron in ch3cn.


/Lewis-dot-structure-58e5390f3df78c5162b4c3db.jpg)